Pioneering
Precision Medicine
Innovative cancer
biomarker technologies
Developed by leading oncology researchers, our innovative technologies offer you unparalleled support for clinical decision-making.


By experts for experts
OCB was created in 2012 by internationally renowned University of Oxford cancer professors, Nick La Thangue and David Kerr to revolutionise personalised medicine using ground-breaking biomarker technology. Our current tests are focussed on colorectal cancer diagnosis, management and treatment; we will be expanding our portfolio of tests to benefit other cancer treatment programmes.
Innovative biomarker platforms
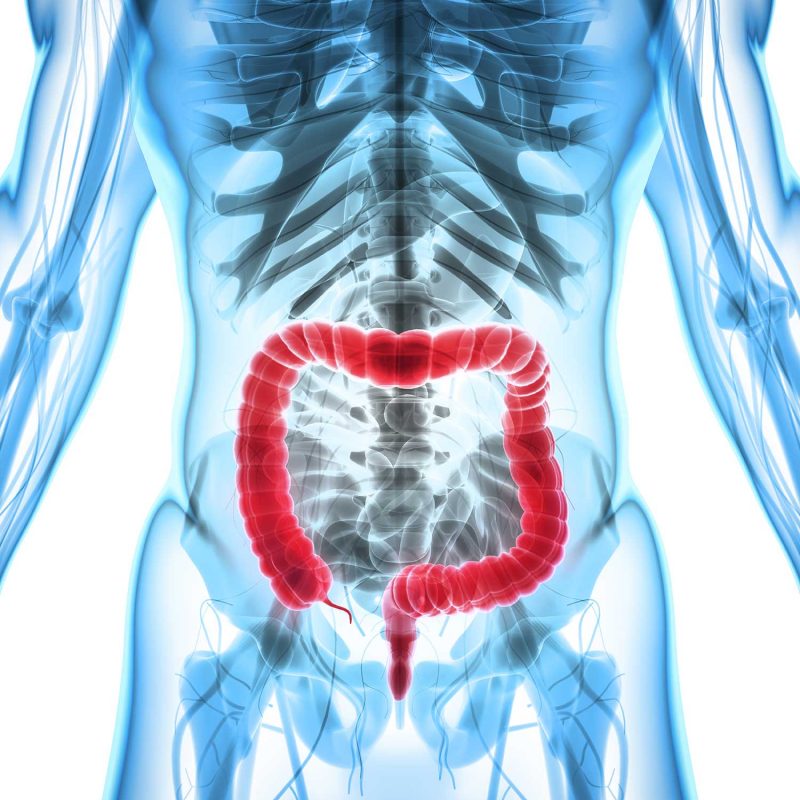

Identify whether your patient is at risk of 5FU/capecitabine toxicity, make more informed treatment decisions and reduce the risk of severe adverse effects with ToxNav. Improve your patients’ quality of life and reduce treatment costs.

What if you could find out which of your patients are at low, intermediate or high risk of colorectal cancer relapse? OncoProg uses innovative digital pathology to create a risk profile for your patients and help inform your next clinical decision.
The future of
precision medicine
We are constantly developing our products to improve their specificity, accuracy and clinical relevance. Read more about our product pipeline or contact us today to find out how you can be a part of our future.
